隨著台灣生技醫藥產業的發展逐步走向國際,對於國外進階課程的需求也日漸增長;為此,台灣光鹽生技學苑國際合作組伍亮霓召集人引薦了多位歐洲的產業專家擔任講師,首先學苑很榮幸地在此宣布- 我們與德國醫藥顧問公司ARC-Traicoa UG (haftungsbeschränkt) 正式合作辦理國際線上同步遠距研討會(Live Webinar)。
對於想了解如何在歐洲開拓臨床試驗領域以及對國際臨床試驗課程感興趣的學員們,你們有福了!
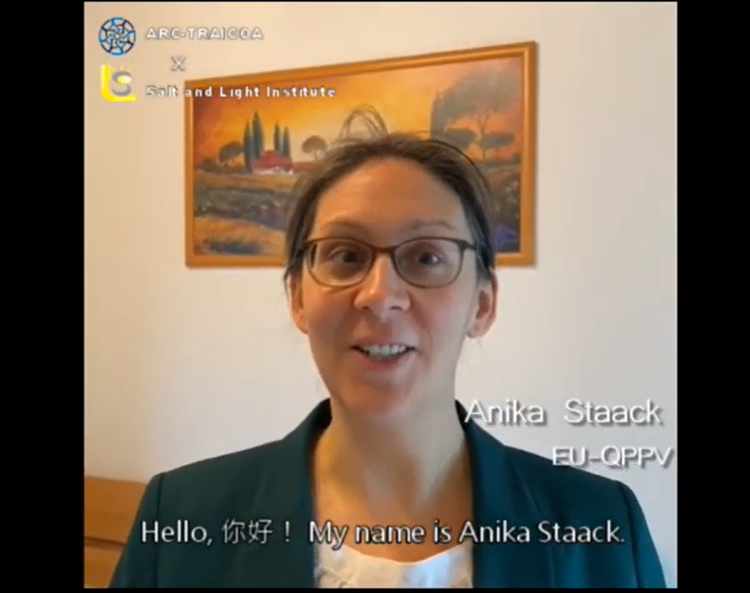
我們的主講者,Anika Staack老師,在EMA及歐洲各國從事臨床試驗領域已有16年餘載的經驗,且身為EU-QPPV的一員,更是醫藥品申請NDA前及上市後不可或缺的角色。希望學員有機會一同參與線上課程,感受國際交流第一手資訊的悸動!
Anika也很親切、熱心地提供了她的一段自我介紹及影片,希望各位能在線上同步遠距研討會(Live Webinar)中與她互動:
“Greetings! My name is Anika Staack. I have more than 16 years of experience in the field of clinical trials and post-marketing of medicinal products. In the past, I had worked as CRA, Trial Site Manager, and Drug Safety Manager for global clinical trials of various indications and patient age groups. For several years now, I have been working as an EU-Qualified Person for Pharmacovigilance (EU-QPPV), overseeing product lifecycle of medicinal products, supplementals, and medical devices. Additionally, I have expertise in a wide array of clinical trial activities, ranging from audit and inspections, Quality Assurance, Regulatory Affairs, Manufacturing, to Marketing. I am knowledgeable about several databases and European pharmaceutical laws and regulations. Moreover, I have been providing training for FORUM Institute about Audits and Inspections and Risk Management. I am thrilled to meet you all in my webinar sessions. See you then!”